pf3 molecular shape|pf3 lewis structure molecular geometry : Tuguegarao A quick explanation of the molecular geometry of PF3 including a description of the PF3 bond angles.Looking at the PF3 Lewis structure we can see that there .
الموسم 1 - شاهدوا أونلاين: بالبث أو الشراء أو التأجير . نسعى باستمرار لإضافة مقدمي خدمة جدد، لكن لم نتمكن من العثور على عرض لمشاهدة "Unicorn: Warriors Eternal - موسم 1" أونلاين.
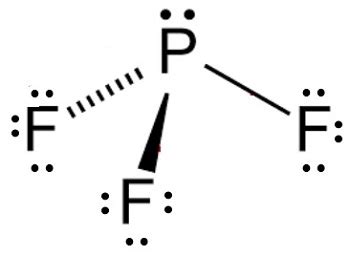
pf3 molecular shape,The geometrical structure of the tetra-atomic Phosphorus Trifluoride (PF3) molecule is studied with the help of the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory explains that the bond angle between the fluorine-phosphorus-fluorine (F-P-F) is 97°. This angle makes . Tingnan ang higit papf3 molecular shape pf3 lewis structure molecular geometryAs per this rule, the maximum number of valence electrons an atom can have is eight. One phosphorus atom has five valence . Tingnan ang higit paThe electrons present in the outermost shell of an atom are called valence electrons. Because they are present in the outermost shell, the hold of the nucleus is weak on . Tingnan ang higit pa
Hybridization is a method of combining atomic orbitals of the same atom to produce new orbitals which are called hybrid orbitals. To figure out the hybridization of the central atom, it is essential . Tingnan ang higit paThe Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. PF3 is a tetra-atomic molecule where phosphorus donates three valence . Tingnan ang higit pa
A quick explanation of the molecular geometry of PF3 including a description of the PF3 bond angles.Looking at the PF3 Lewis structure we can see that there .Phosphorus trifluoride has an F−P−F bond angle of approximately 96.3°. Gaseous PF3 has a standard enthalpy of formation of −945 kJ/mol (−226 kcal/mol). The phosphorus atom has a nuclear magnetic resonance chemical shift of 97 ppm (downfield of H3PO4).
Phosphorus trifluoride (formula PF3), is a colourless and odourless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. In this video, we will.
The molecular geometry or shape for PF3 is the trigonal pyramid. The electron geometry for PF3 is tetrahedral as it central has 4 regions of electron density. Lewis dot structure of PF3 contains 1 lone . PF3 is a covalent molecule where P is located at a central position and surrounded by three F atoms. P and F are covalently bonded along with sp3 .The molecule of phosphorus trifluoride (with trigonal pyramidal shape PF3 molecular geometry) is tilted at 97 degrees bond angle of F-P-F. It has a difference in .Formula: F 3 P. Molecular weight: 87.968972. IUPAC Standard InChI:InChI=1S/F3P/c1-4 (2)3 Copy. IUPAC Standard InChIKey:WKFBZNUBXWCCHG-UHFFFAOYSA-N Copy. . We show you how to draw the Lewis structure and determine the moleculargeometry for phosphorus trifluoride (PF3).
PF 3 (phosphorus trifluoride) has one phosphorus atom and three fluorine atoms. In the PF 3 Lewis structure, there are three single bonds around the phosphorus . Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to .

Answer and Explanation: Become a Study.com member to unlock this answer! Create your account. View this answer. PF 3 has a trigonal pyramidal molecular geometry. The Lewis structure of PF 3 is: Let's count the areas around the . PF3 Molecular Geometry,Shape and Bond Angles (Phosphorous Triflouride) Phosphorus trifluoride (formula PF3), is a colourless and odourless gas. It is highly toxic and reacts slowly .VSEPR & shapes of molecules. Draw the shape of the following molecules: Phosphorus (V) chloride. N (CH 3) 3. CCl 4. Answer 1: Phosphorus is in group 15, so has 5 valence electrons; Cl is in group 17, so has 17 valence electrons. All 5 electrons are used to form covalent bonds with Cl and there are no lone pairs.Figure 8.6.1 8.6. 1 shows the various molecular geometries for the five VESPR electronic geometries with 2 to 6 electron domains. When there are no lone pairs the molecular geometry is the electron (VESPR) geometry. When there are lone pairs, you need to look at the structure and recognize the names and bond angles.
Table 7.3.1 7.3. 1: Different Molecular Geometries. 2 dimensional structure of H 2 O shows a central O bonded to two H forming an overall V shaped structure. In each of the molecules shown in Table 7.3.1 7.3. 1 the electron-pair bonds are arranged so that they avoid each other in space to the maximum possible extent.

Modified by Tom Neils (Grand Rapids Community College) 3.10 Shapes of Molecules - VSEPR Theory and Valence Bond Theory is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Molecular shapes can be predicted from the number of electron pairs attached to each central atom in a . Step #1: Calculate the total number of valence electrons. Here, the given molecule is PF3 (phosphorus trifluoride). In order to draw the lewis structure of PF3, first of all you have to find the total number of valence electrons present in the PF3 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).The Shapes of Molecules. The chemical bonding in a compound is very obviously related to its reactivity and properties – Na2O and H2O being quite different materials. It is perhaps less obvious that the shape of a molecule may also be crucial to its physical and chemical properties. The artificial sweetener, HO. aspartame, appears quite.
pf3 lewis structure molecular geometry PF3 (phosphorus trifluoride) has one phosphorus central atom surrounded by three fluorine atoms and one lone pair of electrons. This gives it a VSEPR notatio. Learn to determine if PF3 (Phosphorous trifluoride) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the L. Notice that a tetrahedral molecule such as CCl4 CCl 4 is nonpolar Figure ( 4.12.1 4.12. 1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ).The molecular geometry of the PF 3 molecule is _____, and this molecule is _____. trigonal pyramidal, nonpolar. trigonal planar, polar. tetrahedral, unipolar. trigonal planar, nonpolar. . Answer : Since PF3 has 3 F group and 1 lone pair its electronic geometry is t .pf3 molecular shapeStep 1. The molecular formula of a molecule is given as PF A 3 . The elements present are P and F. View the full answer Step 2. Unlock. Answer. Unlock. Previous question Next question. Transcribed image text:Phosphorus trifluoride. Phosphorus trifluoride. Formula: F 3 P. Molecular weight: 87.968972. IUPAC Standard InChI:InChI=1S/F3P/c1-4 (2)3 Copy. IUPAC Standard InChIKey:WKFBZNUBXWCCHG-UHFFFAOYSA-N Copy. CAS Registry Number: 7783-55-3.
SHAPES OF MOLECULES AND IONS The shape of a molecule depends upon its electronic structure. It is the outer shell or valence shell electrons which are responsible for forming bonds and it is the arrangement of these electrons which determine molecular shape. The electrons are all negatively charged and so will repel each other.The PF3 molecule’s three P-F bonds are arranged in symmetrical polarity order around the trigonal pyramidal molecular geometry, giving rise to the PF3 molecular shape. The PF3 molecule has a trigonal pyramidal molecular geometry because there is an electrical repulsion between the lone pairs of electrons in phosphorus and three single bond . 8.1.3 Hazards Summary. A strong skin, eye, and mucous membrane irritant; Inhalation my cause pulmonary edema; [Merck Index] A corrosive substance that can cause injury to the skin, eyes, and respiratory tract; Can be absorbed through skin; Inhalation may cause chemical pneumonitis; [MSDSonline] See FLUORIDES.
pf3 molecular shape|pf3 lewis structure molecular geometry
PH0 · pf3 name
PH1 · pf3 lewis structure molecular geometry
PH2 · pf3 axe notation
PH3 · molecular shapes chart
PH4 · br3 molecular geometry
PH5 · Iba pa
PH6 · 3d molecule viewer
PH7 · 3d molecular shapes